Abstract
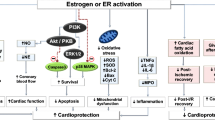
A high proportion of primary percutaneous coronary interventions performed in the setting of acute myocardial infarction, concur with inadequate myocardial perfusion at the microvascular level. This phenomenon, known as “no-reflow” contributes to reperfusion injury, poor prognosis and to unfavorable clinical outcome. In this study, we evaluated the hypothesis that the synthetic 17β-aminoestrogen Prolame, may confer cardioprotection and prevent against no-reflow. In an open-chest model of 30-min ischemia and 90-min reperfusion, male Wistar rats were randomly assigned to different groups: Control, Prolame, Prolame followed by the nitric oxide synthase inhibitor (l-NAME), and 17β-estradiol. Areas of risk, infarct size and no-reflow were determined by planimetry with triphenyltetrazolium chloride and thioflavin-S stains. Structural damage of the vasculature was measured as capillary compression in clarified tissue after intra-atrial injection of Microfil. Hemodynamic function was obtained at the end of stabilization, ischemia and reperfusion; nitric oxide (NO·) content was determined indirectly using the Griess reaction. Activation of the eNOS signaling cascade was determined by western blot. Prolame reduced the infarcted area, decreased the zones of no-reflow and capillary compression by activating the PI3K/Akt/eNOS signaling pathway in correlation with NO· increase. Prolame also activated endothelial cells augmenting NO· production, which was inhibited by ICI182780 (a selective estrogen receptor down-regulator), supporting the notion that the cardioprotective effect of Prolame involves the preservation of endothelium through the activation of estrogen receptor downstream signaling. Our results provide evidence that Prolame has potential therapeutic application in patients with AMI, as it prevents from both vascular and cardiac tissue damage.





Similar content being viewed by others
References
Amit G, Cafri C, Yaroslavtsev S, Fuchs S, Paltiel O, Abu-Ful A, Weinstein JM, Wolak A, Ilia R, Zahger D (2006) Intracoronary nitroprusside for the prevention of the no-reflow phenomenon after primary percutaneous coronary intervention in acute myocardial infarction. A randomized, double-blind, placebo-controlled clinical trial. Am Heart J 152(887):e9–e14. doi:10.1016/j.ahj.2006.05.010
Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA (1990) Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci USA 87:1620–1624. doi:10.1073/pnas.87.4.1620
Brady AJ, Warren JB, Poole-Wilson PA, Williams TJ, Harding SE (1993) Nitric oxide attenuates cardiac myocyte contraction. Am J Physiol 265:H176–H182
Butler MJ, ChanW Taylor AJ, Dart AM, Duffy SJ (2011) Management of the no-reflow phenomenon. Pharmacol Ther 132:72–85. doi:10.1016/j.pharmthera.2011.05.010
Coral-Vazquez RM, Cohn RD, Moore SA, Hill JA, Weiss RM, Davisson RL, Straub V, Barresi R, Bansal D, Hrstka RF, Williamson R, Campbell KP (1999) Disruption of the sarcoglycan-sarcospan complex in vascular smooth muscle: a novel mechanism for cardiomyopathy and muscular dystrophy. Cell 98:465–474. doi:10.1016/S0092-8674(00)81975-3
Fernández-G JM, Rubio-Arroyo MF, Soriano-García M, Toscano RA, Pérez-César MC (1985) Synthesis and molecular structure of prolame, N-(3-hydroxy-1,3,5(10)-estratrien-17 beta-yl)-3-hydroxypropylamine; an amino-estrogen with prolonged anticoagulant and brief estrogenic effects. Steroids 45:151–157
Florian M, Lu Y, Angle M, Magder S (2004) Estrogen induced changes in Akt-dependent activation of endothelial nitric oxide synthase and vasodilation. Steroids 69:637–645. doi:10.1016/j.steroids.2004.05.016
Fokkema ML, Vlaar PJ, Vogelzang M, Gu YL, Kampinga MA, de Smet BJ, Jessurun GA, Anthonio RL, van den Heuvel AF, Tan ES, Zijlstra F (2009) Effect of high-dose intracoronary adenosine administration during primary percutaneous coronary intervention in acute myocardial infarction: a randomized controlled trial. Circ Cardiovasc Interv 2:323–329. doi:10.1161/CIRCINTERVENTIONS.109.858977.109.858977
Fugit MD, Rubal BJ, Donovan DJJ (2000) Effects of intracoronary nicardipine, diltiazem and verapamil on coronary blood flow. Invasive Cardiol 12:80–85
Galasso G, Schiekofer S, D’Anna C, Di Gioia G, Piccolo R, Niglio T, De Rosa R, Strisciuglio T, Cirillo P, Piscione F, Trimarco B (2014) No-reflow-phenomenon: pathophysiology, diagnosis, prevention and treatment. Angiology 65:180–189. doi:10.1177/0003319712474336
Garg UC, Hassid A (1989) Nitric oxide-generating vasodilators and 8-bromo-cyclic guanosine monophosphate inhibit mitogenesis and proliferation of cultured rat vascular smooth muscle cells. J Clin Invest 83:1774–1777. doi:10.1172/JCI114081
González G, Alvarado-Vasquez N, Fernández-G JM, Cruz-Robles D, Del Valle L, Pinzón E, Torres I, Rodriguez E, Zapata E, Gómez-Vidales V, Montaño LF, de la Peña A (2010) The antithrombotic effect of the aminoestrogenprolame (N-(3-hydroxy-1,3,5(10)-estratrien-17B-YL)-3-hydroxypropylamine) is linked to an increase in nitric oxide production by platelets and endothelial cells. Atherosclerosis 208:62–68. doi:10.1016/j.atherosclerosis.2009.06.017
Granger DL, Taintor RR, Boockvar KS, Hibbs JB Jr (1996) Measurement of nitrate and nitrite in biological samples using nitrate reductase and Griess reaction. Methods Enzymol 268:142–151
Grines CL, Westerhausen DR Jr, Grines LL, Hanlon JT, Logemann TL, Niemela M, Weaver WD, Graham M, Boura J, O’Neill WW, Balestrini C, Air PAMI Study Group (2002) A randomized trial of transfer for primary angioplasty versus on-site thrombolysis in patients with high-risk myocardial infarction: the air primary angioplasty in myocardial infarction study. J Am Coll Cardiol 39:1713–1719. doi:10.1016/S0735-1097(02)01870-3
Gross SS, Wolin MS (1995) Nitric oxide: pathophysiological mechanisms. Ann Rev Physiol 57:737–769. doi:10.1146/annurev.ph.57.030195.003513
Hale SL, Herring MJ, Kloner RA (2013) Delayed treatment with hypothermia protects against the no-reflow phenomenon despite failure to reduce infarct size. J Am Heart Assoc 2:e004234. doi:10.1161/JAHA.112.004234
Hale SL, Mehra A, Leeka J, Kloner RA (2008) Postconditioning fails to improve no reflow or alter infarct size in an open-chest rabbit model of myocardial ischemia-reperfusion. Am J Physiol Heart Circ Physiol 294:H421–H425
Haynes MP, Li L, Sinha D, Russell KS, Hisamoto K, Baron R, Collinge M, Sessa WC, Bender JR (2003) Src kinase mediates phosphatidylinositol 3-kinase/Akt-dependent rapid endothelial nitric-oxide synthase activation by estrogen. J Biol Chem 278:2118–2123. doi:10.1074/jbc.M210828200
Herring MJ, Dai W, Hale SL, Kloner RA (2014) Rapid induction of hypothermia by the ThermoSuit system profoundly reduces infarct size and anatomic zone of no reflow following ischemia-reperfusion in rabbit and rat hearts. J Cardiovasc Pharmacol Ther (in press) pii: 1074248414535664
Heusch G (2010) Adenosine and maximum coronary vasodilation in humans: myth and misconceptions in the assessment of coronary reserve. Basic Res Cardiol 105:1–5. doi:10.1007/s00395-009-0074-7
Heusch G (2013) Cardioprotection: chances and challenges of its translation to the clinic. Lancet 381:166–175. doi:10.1016/S0140-6736(12)60916-7
Heusch G, Kleinbongard P, Böse D, Levkau B, Haude M, Schulz R, Erbel R (2009) Coronary microembolization: from bedside to bench and back to bedside. Circulation 120:1822–1836. doi:10.1161/CIRCULATIONAHA.109.888784
Heusch G, Kleinbongard P, Skyschally A (2013) Myocardial infarction and coronary microvascular obstruction: an intimate, but complicated relationship. Basic Res Cardiol 108:380–382. doi:10.1007/s00395-013-0380-y
Ignarro LJ, Buga GM, Wood KS, Byrns RE, Chaudhuri G (1987) Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci USA 84:9265–9269. doi:10.1073/pnas.84.24.9265
Iwakura K, Ito H, Kawano S, Okamura A, Kurotobi T, Date M, Inoue K, Fujii K (2006) Chronic pre-treatment of statins is associated with the reduction of the no-reflow phenomenon in the patients with reperfused acute myocardial infarction. Eur Heart J 27:534–539. doi:10.1093/eurheartj/ehi715
Jaimez R, Cooney A, Jackson K, Lemus AE, Lemini C, Cárdenas M, García R, Silva G, Larrea F (2000) In vivo estrogen bioactivities and in vitro estrogen receptor binding and transcriptional activities of anticoagulant synthetic 17beta-aminoestrogens. J Steroid Biochem Mol Biol 73:59–66. doi:10.1016/S0960-0760(00)00053-4
Kalinowski L, Matys T, Chabielska E, Buczko W, Malinski T (2002) Angiotensin II AT1 receptor antagonists inhibit platelet adhesion and aggregation by nitric oxide release. Hypertension 40:521–527. doi:10.1161/01.HYP.0000034745.98129.EC
Keeley EC, Boura JA, Grines C (2003) Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet 361:13–20. doi:10.1016/S0140-6736(03)12113-7
Kloner RA, Ganote CE, Jennings RB (1974) The “no-reflow” phenomenon after temporary coronary occlusion in the dog. J Clin Invest 54:1496–1508. doi:10.1172/JCI107898
Lee CH, Wong HB, Tan HC, Zhang JJ, Teo SG, Ong HY, Low A, Sutandar A, Lim YT (2005) Impact of reversibility of no reflow phenomenon on 30-day mortality following percutaneous revascularization for acute myocardial infarction-insights from a 1,328 patient registry. J Interv Cardiol 18:261–266. doi:10.1111/j.1540-8183.2005.00041.x
Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, Chambers CE, Ellis SG, Guyton RA, Hollenberg SM, Khot UN, Lange RA, Mauri L, Mehran R, Moussa ID, Mukherjee D, Nallamothu BK, Ting HH, ACCF, AHA, SCAI (2011) 2011 ACCF/AHA/SCAI Guideline for percutaneous coronary intervention: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. J Am Coll Cardiol 58:e44–e122. doi:10.1016/j.jacc.2011.08.007
Li XD, Yang YJ, Geng YJ, Zhang HT, Cheng YT, Wu YL (2012) Phosphorylation of endothelial NOS contributes to simvastatin protection against myocardial no-reflow and infarction in reperfused swine hearts: partially via the PKA signaling pathway. Acta Pharmacol Sin 33:879–887. doi:10.1038/aps
Loke KE, McConnell P, Tuzman JM, Shesely EG, Smith CJ, Stackpole CJ, Thompson CI, Kaley G, Wolin MS, Hintze T (1999) Endogenous endothelial nitric oxide synthase-derived nitric oxide is a physiological regulator of myocardial oxygen consumption. Circ Res 84:840–845. doi:10.1161/01.RES.84.7.840
Ma XL, Weyrich AS, Lefer DJ, Lefer AM (1993) Diminished basal nitric oxide release after myocardial ischemia and reperfusion promotes neutrophil adherence to coronary endothelium. Circ Res 72:403–412. doi:10.1161/01.RES.72.2.403
Mahaffey KW, Puma JA, Barbagelata NA, DiCarli MF, Leesar MA, Browne KF, Eisenberg PR, Bolli R, Casas AC, Molina-Viamonte V, Orlandi C, Blevins R, Gibbons RJ, Califf RM, Granger CB (1999) Adenosine as an adjunct to thrombolytic therapy for acute myocardial infarction: results of a multicenter, randomized, placebo-controlled trial: the Acute Myocardial Infarction STudy of ADenosine (AMISTAD) trial. J Am Coll Cardiol 34:1711–1720. doi:10.1016/S0735-1097(99)00418-0
Mandoki JJ, Zavala E, Silva G, Mendoza-Patiño N, Rubio-Póo C, Medina-Martínez S, Domínguez-Escoto P (1983) The dual effects of estrogens on blood clotting time. Proc West Pharmacol Soc 26:45–48
Murphy E (2011) Estrogen signaling and cardiovascular disease. Circ Res 109:687–696. doi:10.1161/CIRCRESAHA.110.236687
Niccoli G, Burzotta F, Galiuto L, Crea F (2009) Myocardial no-reflow in humans. J Am Coll Cardiol 54:281–292. doi:10.1016/j.jacc.2009.03.054
O´Farrell F, Attwell D (2014) A role for pericytes in coronary no-reflow. Nat Rev Cardiol 7:427–432. doi:10.1038/nrcardio.2014.58
Resnic FS, Wainstein M, Lee MK, Behrendt D, Wainstein RV, Ohno-Machado L, Kirshenbaum JM, Rogers CD, Popma JJ, Piana R (2003) No-reflow is an independent predictor of death and myocardial infarction after percutaneous coronary intervention. Am Heart J 145:42–46. doi:10.1067/mhj.2003.36
Ross AM, Gibbons RJ, Stone GW, Kloner RA, Alexander RW, AMISTAD-II Investigators (2005) A randomized, double-blinded, placebo-controlled multicenter trial of adenosine as an adjunct to reperfusion in the treatment of acute myocardial infarction (AMISTAD-II). J Am Coll Cardiol 45:1775–1780. doi:10.1016/j.jacc.2005.02.061
Roubille F, Lairez O, Mewton N, Rioufol G, Ranc S, Sanchez I, Cung TT, Elbaz M, Piot C, Ovize M (2012) Cardioprotection by clopidogrel in acute ST-elevated myocardial infarction patients: a retrospective analysis. Basic Res Cardiol 107:275. doi:10.1007/s00395-012-0275-3
Roubille F, Tardif J-C (2013) Cardioprotection—time to take into account clinical complexity: the case of antiplatelet agents. Cardiovasc Drugs Ther 27:105–107. doi:10.1007/s10557-013-6443-3
Salarifar M, Mousavi MR, Saroukhani S, Nematipour E, Kassaian SE, Alidoosti M, Poorhosseini HR, Haji-Zeinali AM, Nozari Y, Hosseini K, Jalali A (2014) Percutaneous coronary intervention to treat chronic total occlusion: predictors of technical success and one-year clinical outcome. Tex Heart Inst J 41:40–47. doi:10.14503/THIJ-12-2731
Sastry KV, Moudgal RP, Mohan J, Tyaqi JS, Rao GS (2002) Spectrophotometric determination of serum nitrite and nitrate by cooper-cadmium alloy. Anal Biochem 306:79–82. doi:10.1006/abio.2002.5676
Schäfer A, Fraccarollo D, Pförtsch S, Loch E, Neuser J, Vogt C, Bauersachs J (2011) Clopidogrel improves endothelial function and NO bioavailability by sensitizing adenylyl cyclase in rats with congestive heart failure. Basic Res Cardiol 106:485–494. doi:10.1007/s00395-011-0153-4
Schwartz LM, Lagranha CJ (2006) Ischemic postconditioning during reperfusion activates Akt and ERK without protecting against lethal myocardial ischemia-reperfusion injury in pigs. Am J Physiol Heart CircPhysiol 290:H1011–H1018. doi:10.1007/s00395-014-0436-7
Skyschally A, van Caster P, Boengler K, Gres P, Musiolik J, Schilawa D, Schulz R, Heusch G (2009) Ischemic postconditioning in pigs: no causal role for RISK activation. Circ Res 104:15–18. doi:10.1161/CIRCRESAHA.108.186429
Werner GS, Lang K, Kuehnert H, Figulla H (2002) Intracoronary verapamil for reversal of no-Reflow during coronary angioplasty for acute myocardial infarction. Catheter Cardiovasc Interv 57:444–451. doi:10.1002/ccd.10375
Yang X-M, Liu Y, Cui L, Yang X, Liu Y, Tandon N, Kambayashi J, Downey JM, Cohen MV (2013) Platelet P2Y12 Blockers Confer Direct Postconditioning-like Protection in Reperfused Rabbit Hearts. J Cardiovasc Pharmacol Ther 18:251–262. doi:10.1177/1074248412467692
Yang XM, Liu Y, Cui L, Yang X, Liu Y, Tandon N, Kambayashi J, Downey JM, Cohen MV (2013) Two classes of anti-platelet drugs reduce anatomical infarct size in monkey hearts. Cardiovasc Drugs Ther 27:109–115. doi:10.1007/s10557-012-6436-7
Zhao JL, Yang YJ, Cui CJ, You SJ, Wu YJ, Gao RL (2006) Different effects of adenosine and calcium channel blockade on myocardial no-reflow after acute myocardial infarction and reperfusion. Cardiovasc Drugs Ther 20:167–175. doi:10.1007/s10557-006-8284-9
Acknowledgments
This article is part of the doctoral thesis of Sauri Hernández-Reséndiz in the Biomedical Sciences Doctoral Program, School of Medicine, National Autonomous University of Mexico (UNAM). Sauri Hernández-Reséndiz received a scholarship (32006) from the National Council of Science and Technology (CONACYT). This work was partially supported by Grant 177527 to CZ from CONACYT, Mexico.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
All authors in this work gave their informed consent prior to their inclusion in the study. The manuscript does not contain clinical studies or patient data.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hernández-Reséndiz, S., Palma-Flores, C., De los Santos, S. et al. Reduction of no-reflow and reperfusion injury with the synthetic 17β-aminoestrogen compound Prolame is associated with PI3K/Akt/eNOS signaling cascade. Basic Res Cardiol 110, 1 (2015). https://doi.org/10.1007/s00395-015-0464-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00395-015-0464-y