Abstract
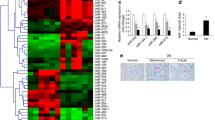
Diabetic nephropathy (DN) is the major cause of chronic kidney disease in developed countries and contributes significantly to increased morbidity and mortality among diabetic patients. Morphologically, DN is characterized by tubulo-interstitial fibrosis, thickening of the glomerular basement membrane and mesangial expansion mainly due to accumulation of extracellular matrix (ECM). ECM turnover is regulated by metalloproteinases and tissue inhibitors of metalloproteinases (TIMPs) activities. In diabetic conditions, TIMP3 expression in kidney is strongly reduced, but the causes of this reduction are still unknown. The aim of this study was to elucidate at least one of these mechanisms which relies on differential expression of TIMP3-targeting microRNAs (miRs) in a hyperglycemic environment either in vitro (MES13 cell line) or in vivo (mouse kidney and human biopsies). Among the TIMP3-targeting miRs, miR-21 and miR-221 were significantly upregulated in kidneys from diabetic mice compared to control littermates, and in a mesangial cell line grown in high glucose conditions. In human samples, only miR-21 expression was increased in kidney biopsies from diabetic patients compared to healthy controls. The expression of miR-217, which targets TIMP3 indirectly through downregulation of SirT1, was also increased in diabetic kidney and MES13 cell line. In agreement with these result, SirT1 expression was reduced in mouse and human diabetic kidneys as well as in MES13 mesangial cell line. TIMP3 deficiency has recently emerged as a hallmark of DN in mouse and human. In this study, we demonstrated that this reduction is due, at least in part, to increased expression of certain TIMP3-targeting miRs in diabetic kidneys compared to healthy controls. Unveiling the post-transcriptional mechanisms responsible for TIMP3 downregulation in hyperglycemic conditions may orient toward the use of this protein as a possible therapeutic target in DN.


Similar content being viewed by others
Abbreviations
- TIMP:
-
Tissue inhibitor of metalloproteinases
- DN:
-
Diabetic nephropathy
- STZ:
-
Streptozotocin
- miR:
-
MicroRNA
- PCR:
-
Polymerase chain reaction
References
Ford BM, Eid AA, Gooz M, Barnes JL, Gorin YC, Abboud HE (2013) ADAM17 mediates Nox4 expression and NADPH oxidase activity in the kidney cortex of OVE26 mice. Am J Physiol Renal Physiol. doi:10.1152/ajprenal.00522.2012
Basu R, Lee J, Wang Z et al (2012) Loss of TIMP3 selectively exacerbates diabetic nephropathy. Am J Physiol Renal Physiol 303:F1341–F1352
Chodavarapu H, Grobe N, Somineni HK, Salem ES, Madhu M, Elased KM (2013) Rosiglitazone treatment of type 2 diabetic db/db mice attenuates urinary albumin and angiotensin converting enzyme 2 excretion. PLoS One 8:e62833
Kassiri Z, Oudit GY, Kandalam V et al (2009) Loss of TIMP3 enhances interstitial nephritis and fibrosis. J Am Soc Nephrol 20:1223–1235
Kawamoto H, Yasuda O, Suzuki T et al (2006) Tissue inhibitor of metalloproteinase-3 plays important roles in the kidney following unilateral ureteral obstruction. Hypertens Res 29:285–294
Federici M, Hribal ML, Menghini R et al (2005) Timp3 deficiency in insulin receptor-haploinsufficient mice promotes diabetes and vascular inflammation via increased TNF-alpha. J Clin Investig 115:3494–3505
Fiorentino L, Vivanti A, Cavalera M et al (2010) Increased tumor necrosis factor alpha-converting enzyme activity induces insulin resistance and hepatosteatosis in mice. Hepatology (Baltimore, MD) 51:103–110
Menghini R, Menini S, Amoruso R et al (2009) Tissue inhibitor of metalloproteinase 3 deficiency causes hepatic steatosis and adipose tissue inflammation in mice. Gastroenterology 136(663–672):e664
Cardellini M, Menghini R, Martelli E et al (2009) TIMP3 is reduced in atherosclerotic plaques from subjects with type 2 diabetes and increased by SirT1. Diabetes 58:2396–2401
Cardellini M, Menghini R, Luzi A et al (2011) Decreased IRS2 and TIMP3 expression in monocytes from offspring of type 2 diabetic patients is correlated with insulin resistance and increased intima-media thickness. Diabetes 60:3265–3270
Menghini R, Casagrande V, Menini S et al (2012) TIMP3 overexpression in macrophages protects from insulin resistance, adipose inflammation, and nonalcoholic fatty liver disease in mice. Diabetes 61:454–462
Casagrande V, Menghini R, Menini S et al (2012) Overexpression of tissue inhibitor of metalloproteinase 3 in macrophages reduces atherosclerosis in low-density lipoprotein receptor knockout mice. Arterioscler Thromb Vasc Biol 32:74–81
Menghini R, Uccioli L, Vainieri E et al (2013) Expression of tissue inhibitor of metalloprotease 3 is reduced in ischemic but not neuropathic ulcers from patients with type 2 diabetes mellitus. Acta Diabetol. doi:10.1007/s00592-013-0478-6
Gohda T, Niewczas MA, Ficociello LH et al (2012) Circulating TNF receptors 1 and 2 predict stage 3 CKD in type 1 diabetes. J Am Soc Nephrol 23:516–524
Niewczas MA, Gohda T, Skupien J et al (2012) Circulating TNF receptors 1 and 2 predict ESRD in type 2 diabetes. J Am Soc Nephrol 23:507–515
Fernandez-Real JM, Vendrell J, Garcia I, Ricart W, Valles M (2012) Structural damage in diabetic nephropathy is associated with TNF-alpha system activity. Acta Diabetol 49:301–305
Fiorentino L, Cavalera M, Menini S et al (2013) Loss of TIMP3 underlies diabetic nephropathy via FoxO1/STAT1 interplay. EMBO Mol Med 5:441–455
Kong L, Zhu J, Han W et al (2011) Significance of serum microRNAs in pre-diabetes and newly diagnosed type 2 diabetes: a clinical study. Acta Diabetol 48:61–69
Saal S, Harvey SJ (2009) MicroRNAs and the kidney: coming of age. Curr Opin Nephrol Hypertens 18:317–323
Kantharidis P, Wang B, Carew RM, Lan HY (2011) Diabetes complications: the microRNA perspective. Diabetes 60:1832–1837
Alvarez ML, DiStefano JK (2013) Towards microRNA-based therapeutics for diabetic nephropathy. Diabetologia 56:444–456
Kato M, Putta S, Wang M et al (2009) TGF-beta activates Akt kinase through a microRNA-dependent amplifying circuit targeting PTEN. Nat Cell Biol 11:881–889
Gabriely G, Wurdinger T, Kesari S et al (2008) MicroRNA 21 promotes glioma invasion by targeting matrix metalloproteinase regulators. Mol Cell Biol 28:5369–5380
Lu Y, Roy S, Nuovo G et al (2011) Anti-microRNA-222 (anti-miR-222) and -181B suppress growth of tamoxifen-resistant xenografts in mouse by targeting TIMP3 protein and modulating mitogenic signal. J Biol Chem 286:42292–42302
Menghini R, Casagrande V, Cardellini M et al (2009) MicroRNA 217 modulates endothelial cell senescence via silent information regulator 1. Circulation 120:1524–1532
Acknowledgments
This work was supported by JDRF RRG 1-2007-665 and Fondazione Roma 2008, PRIN 2009FATXW3_002 (all to M.F.) and Ministero della Salute-Ricerca Finalizzata 2008 to L.G.
Conflict of interest
The authors declare that there is no duality of interest associated with this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Renato Lauro.
Rights and permissions
About this article
Cite this article
Fiorentino, L., Cavalera, M., Mavilio, M. et al. Regulation of TIMP3 in diabetic nephropathy: a role for microRNAs. Acta Diabetol 50, 965–969 (2013). https://doi.org/10.1007/s00592-013-0492-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-013-0492-8