Abstract
Background
Despite the significance of hypertrophy of the ligamentum flavum (HLF) in the disease progress of neurogenic claudication, the cellular mechanisms underlying the gradual fibrotic thickening of the ligamentum flavum remain poorly understood. The aim of our study was to get insight into the contribution of inflammatory mechanisms to the development of hypertrophy.
Methods
Specimens of hypertrophied ligamenta flava were obtained at surgery from 20 patients with acquired lumbar osteoligamentous spinal canal stenosis from the central part of the ligament. Paraffin sections were stained with hematoxylin and eosin and Elastica van Gieson to evaluate extracellular matrix architecture, and immunohistochemistry was performed to characterize the inflammatory reaction and the sources of transforming growth factor beta (TGF-β) expression. Sections of normal ligamenta flava obtained from corresponding anatomical sites and stained in parallel served as a control.
Results
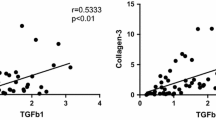
HLF was characterized by a considerable distortion of the elastic matrix and fibrotic transformation by extracellular collagen deposition. All specimens showed highly inflammatory cellular infiltrates confined to regions exhibiting marked degeneration of the elastic matrix composed mainly of macrophages, scattered T lymphocytes, and neovascularization, thus representing a chronic inflammation. Surprisingly, macrophages as well as vascular endothelial cells but not fibroblasts showed a strong expression of TGF-β, a strong inducer of extracellular collagen deposition.
Conclusions
Macrophages were identified as a major cellular source of TGF-β in advanced HLF and may perpetuate further hypertrophy. This finding suggests that modulating the immune response locally or systemically could prove to be effective for impeding the disease progress.



Similar content being viewed by others
References
Adair-Kirk TL, Senior RM (2008) Fragments of extracellular matrix as mediators of inflammation. Int J Biochem Cell Biol 40:1001–1110
Benoist M (2003) Natural history of the aging spine. Eur Spine J 12(suppl 2):S86–S89
Cutroneo KR, Sterling KM Jr (2004) How do glucocorticoids compare to sense oligos containing TGF-β element as inhibitors of collagen synthesis? J Cell Biochem 92:6–15
Cutroneo KR (2007) TGF-β-induced fibrosis and SMAD signaling: oligo decoys as natural therapeutics for inhibition of tissue fibrosis and scarring. Wound Repair Regen 15:54–60
Dockerty MB, Love JG (1940) Thickening and fibrosis (so-called hypertrophy) of the ligamentum flavum: a pathologic study of fifty cases. Proc Staff Meet Mayo Clin 15:161–166
Duca L, Blanchevoye C, Cantarelli B, Ghoneim C, Dedieu S, Delacoux F, Hornebeck W, Hinek A, Martiny L, Debelle L (2007) The elastin receptor complex transduces signals through the catalytic activity of its Neu-1 subunit. J Biol Chem 282:12484–12491
Duftner C, Seiler R, Dejaco-C FG, Schirmer M (2006) Increasing evidence for immune-mediated processes and new therapeutic approaches in abdominal aortic aneurysms—a review. Ann NY Acad Sci 1085:331–338
Frank S, Madlener M, Werner S (1996) Transforming growth factors β1, β2, and β3 and their receptors are differentially regulated during normal and impaired wound healing. J Biol Chem 271:10188–10193
Fukuyama S, Nakamura T, Ikeda T, Takagi K (1995) The effect of mechanical stress on hypertrophy of the lumbar ligamentum flavum. J Spinal Disord 8:126–130
Gauss-Müller V, Kleinman HK, Martin GR, Schiffman E (1980) Role of attachment factors and attractants in fibroblast chemotaxis. J Lab Clin Med 96:1071–1080
Hantash BM, Zhao L, Knowles JA, Lorenz HP (2008) Adult and fetal wound healing. Front Biosci 13:51–61
Houghton AM, Quintero PA, Perkins DL, Kobayashi DK, Kelley DG, Marconcini LA, Mecham RP, Senior RM, Shapiro SD (2006) Elastin fragments drive disease progression in a murine model of emphysema. J Clin Invest 116:753–759
Hunninghake GW, Davidson JM, Rennard S, Szapiel S, Gadek JE, Crystal RG (1981) Elastin fragments attract macrophage precursors to diseased sites in pulmonary emphysema. Science 212:925–926
Kelleher CM, McLean SE, Mecham RP (2004) Vascular extracellular matrix and aortic development. Curr Top Dev Biol 62:153–188
Kosaka H, Sairyo K, Biyani A, Leaman D, Yeasting R, Higashino K, Sakai T, Katoh S, Sano T, Goel VK, Yasui N (2007) Pathomechanism of loss of elasticity and hypertrophy of lumbar ligamentum flavum in elderly patients with lumbar spinal canal stenosis. Spine 32:2805–2811
Larbi A, Levesque G, Robert L, Gagné D, Douziech N, Fülöp T (2005) Presence and active synthesis of the 67 kDa elastin-receptor in human circulating white blood cells. Biochem Biophys Res Commun 332:787–792
Marui T, Niyibizi C, Georgescu HI, Cao M, Kavalkovich KW, Levine RE, Woo SL (1997) Effect of growth factors on matrix synthesis by ligament fibroblasts. J Orthop Res 15:18–23
Nachemson AL, Evans JH (1968) Some mechanical properties of the third human lumbar interlaminar ligament (ligamentum flavum). J Biomech 1:211–220
Nakatani T, Marui T, Hitora T, Doita M, Nishida K, Kurosaka M (2002) Mechanical stretching force promotes collagen synthesis by cultured cells from human ligamentum flavum via transforming growth factor-β1. J Orthop Res 20:1380–1386
Okuda T, Baba I, Fujimoto Y, Tanaka N, Sumida T, Manabe H, Hayashi Y, Ochi M (2004) The pathology of ligamentum flavum in degenerative lumbar disease. Spine 29:1689–1697
Park JB, Lee JK, Park SJ, Riew D (2005) Hypertrophy of ligamentum flavum in lumbar spinal stenosis associated with increased proteinase inhibitor concentration. J Bone Joint Surg Am 87:2750–2757
Postacchini F, Gumina S, Cinotti G, Perugia D, DeMartino C (1994) Ligamenta flava in lumbar disc herniation and spinal stenosis. Spine 19:917–922
Privitera S, Prody CA, Callahan JW, Hinek A (1998) The 67-kDa enzymatically inactive alternatively spliced variant of beta-galactosidase is identical to the elastin/laminin-binding protein. J Biol Chem 273:6319–6326
Ramani PS, Perry RH, Tomlinson BE (1975) Role of ligamentum flavum in the symptomatology of prolapsed lumbar intervertebral discs. J Neurol Neurosurg Psychiatry 38:550–557
Ramsay RH (1966) The anatomy of the ligamenta flava. Clin Orthop 44:129–140
Rhett JM, Ghatnekar GS, Palatinus JA, O’Quinn M, Yost MJ, Gourdie RG (2008) Novel therapies for scar reduction and regenerative healing of skin wounds. Trends Biotechnol 26:173–180
Ruiz-Ortega M, Rodríguez-Vita J, Sanchez-Lopez E, Carvajal G, Egido J (2007) TGF-β signaling in vascular fibrosis. Cardiovasc Res 74:196–206
Sairyo K, Biyani A, Goel V, Leaman D, Booth R, Thomas J, Gehling D, Vishnubhotla SL, Long R, Ebraheim N (2005) Pathomechanism of ligamentum flavum hypertrophy: a multidisciplinary investigation based on clinical, biomechanical, histologic, and biologic assessments. Spine 30:2649–2656
Sairyo K, Biyani A, Goel VK, Leaman DW, Booth R, Thomas J, Ebraheim NA, Cowgill IA, Mohan SE (2007) Lumbar ligamentum flavum hypertrophy is due to accumulation of inflammation-related scar tissue. Spine 32:E340–E347
Senior RM, Griffin GL, Mecham RP (1980) Chemotactic activity of elastin-derived peptides. J Clin Invest 66:859–862
Senior RM, Griffin GL, Mecham RP (1982) Chemotactic responses of fibroblasts to tropoelastin and elastin-derived peptides. J Clin Invest 70:614–618
Senior RM, Griffin GL, Mecham RP, Wrenn DS, Prasad KU, Urry DW (1984) Val-Gly-Val-Ala-Pro-Gly, a repeating peptide in elastin, is chemotactic for fibroblasts and monocytes. J Cell Biol 99:870–874
Stramer BM, Mori R, Martin P (2007) The inflammation–fibrosis link? A Jekyll and Hyde role for blood cells during wound repair. J Invest Dermatol 127:1009–1017
Szpalski M, Gunzburg R (2003) Lumbar spinal stenosis in the elderly: an overview. Eur Spine J 12(suppl 2):170–175
Verbiest H (1954) A radicular syndrome from developmental narrowing of the lumbar vertebral canal. J Bone Joint Surg Br 36:230–237
Verrecchia F, Mauviel A (2002) Transforming growth factor-beta signaling through the Smad pathway: role in extracellular matrix gene expression and regulation. J Invest Dermatol 118:211–215
Yahia LH, Garzon S, Strykowski H, Rivard CH (1990) Ultrastructure of the human interspinous ligament and ligamentum flavum. Spine 15:262–268
Yoshida M, Shima K, Taniguchi Y, Tamaki T, Tanaka T (1992) Hypertrophied ligamentum flavum in lumbar spinal canal stenosis. Spine 17:1353–1360
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Löhr, M., Hampl, J.A., Lee, J.Y. et al. Hypertrophy of the lumbar ligamentum flavum is associated with inflammation-related TGF-β expression. Acta Neurochir 153, 134–141 (2011). https://doi.org/10.1007/s00701-010-0839-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-010-0839-7