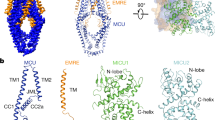
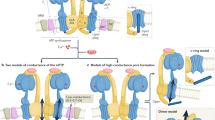
Key Points
-
The molecular identity of the human mitochondrial calcium uniporter has now been elucidated using integrative genomics. The mammalian uniporter is a protein complex consisting of the inner membrane-spanning subunits mitochondrial calcium uniporter protein (MCU), MCUb and essential MCU regulator (EMRE), together with mitochondrial calcium uptake protein 1 (MICU1) and MICU2 in the intermembrane space.
-
Expression of human MCU and EMRE is sufficient to reconstitute uniporter pore activity in vivo.
-
MICU1 and MICU2 operate together in the intermembrane space to sense calcium concentration and regulate the uniporter.
-
Loss of uniporter activity can be tolerated in vivo in organisms including trypanosomes, worms and mice.
-
Genetic studies in cells and in model organisms are converging on a key function for this channel in the coupling of cellular ATP consumption with its production (termed excitation–energetic coupling). The role of the channel in cell death and apoptosis remains controversial.
-
Inherited mutations in the uniporter machinery have been identified in patients with neuromuscular disease.
-
Next steps include using structural biology to gain mechanistic insights into the uniporter and investigating pathologies associated with the manipulation of uniporter activity, which may suggest a therapeutic strategy for targeting the uniporter.
Abstract
The mitochondrial calcium uniporter is an evolutionarily conserved calcium channel, and its biophysical properties and relevance to cell death, bioenergetics and signalling have been investigated for decades. However, the genes encoding this channel have only recently been discovered, opening up a new 'molecular era' in the study of its biology. We now know that the uniporter is not a single protein but rather a macromolecular complex consisting of pore-forming and regulatory subunits. We review recent studies that harnessed the power of molecular biology and genetics to characterize the mechanism of action of the uniporter, its evolution and its contribution to physiology and human disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Berridge, M. J., Bootman, M. D. & Lipp, P. Calcium—a life and death signal. Nature 395, 645–648 (1998).
Deluca, H. F. & Engstrom, G. W. Calcium uptake by rat kidney mitochondria. Proc. Natl Acad. Sci. USA 47, 1744–1750 (1961).
Vasington, F. D. & Murphy, J. V. Ca ion uptake by rat kidney mitochondria and its dependence on respiration and phosphorylation. J. Biol. Chem. 237, 2670–2677 (1962).
Gunter, T. E. & Pfeiffer, D. R. Mechanisms by which mitochondria transport calcium. Am. J. Physiol. 258, C755–C786 (1990).
Kirichok, Y., Krapivinsky, G. & Clapham, D. E. The mitochondrial calcium uniporter is a highly selective ion channel. Nature 427, 360–364 (2004). This study used mitoplast electrophysiology to rigorously characterize the uniporter current, showing that the uniporter is a highly selective calcium channel with high conductance.
Palty, R. et al. NCLX is an essential component of mitochondrial Na+/Ca2+ exchange. Proc. Natl Acad. Sci. USA 107, 436–441 (2010).
Jiang, D., Zhao, L. & Clapham, D. E. Genome-wide RNAi screen identifies Letm1 as a mitochondrial Ca2+/H+ antiporter. Science 326, 144–147 (2009).
Denton, R. M. & McCormack, J. G. The role of calcium in the regulation of mitochondrial metabolism. Biochem. Soc. Trans. 8, 266–270 (1980).
Hajnoczky, G., Robb-Gaspers, L. D., Seitz, M. B. & Thomas, A. P. Decoding of cytosolic calcium oscillations in the mitochondria. Cell 82, 415–424 (1995).
Jouaville, L. S., Ichas, F., Holmuhamedov, E. L., Camacho, P. & Lechleiter, J. D. Synchronization of calcium waves by mitochondrial substrates in Xenopus laevis oocytes. Nature 377, 438–441 (1995).
Zong, W. X. & Thompson, C. B. Necrotic death as a cell fate. Genes Dev. 20, 1–15 (2006).
Duchen, M. R. Mitochondria and calcium: from cell signalling to cell death. J. Physiol. 529, 57–68 (2000).
Ma, J. Block by ruthenium red of the ryanodine-activated calcium release channel of skeletal muscle. J. Gen. Physiol. 102, 1031–1056 (1993).
Cibulsky, S. M. & Sather, W. A. Block by ruthenium red of cloned neuronal voltage-gated calcium channels. J. Pharmacol. Exp. Ther. 289, 1447–1453 (1999).
Pagliarini, D. J. et al. A mitochondrial protein compendium elucidates complex I disease biology. Cell 134, 112–123 (2008).
Carafoli, E. & Lehninger, A. L. A survey of the interaction of calcium ions with mitochondria from different tissues and species. Biochem. J. 122, 681–690 (1971).
Docampo, R. & Vercesi, A. E. Ca2+ transport by coupled Trypanosoma cruzi mitochondria in situ. J. Biol. Chem. 264, 108–111 (1989).
Perocchi, F. et al. MICU1 encodes a mitochondrial EF hand protein required for Ca2+ uptake. Nature 467, 291–296 (2010). This article reports the identification of the first uniporter molecular component, MICU1, which paved the way for the identification of the rest of the complex.
Baughman, J. M. et al. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature 476, 341–345 (2011).
De Stefani, D., Raffaello, A., Teardo, E., Szabo, I. & Rizzuto, R. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature 476, 336–340 (2011). References 19 and 20 identify MCU, which is the pore-forming subunit of the uniporter.
Plovanich, M. et al. MICU2, a paralog of MICU1, resides within the mitochondrial uniporter complex to regulate calcium handling. PLoS ONE 8, e55785–e55785 (2013).
Raffaello, A. et al. The mitochondrial calcium uniporter is a multimer that can include a dominant-negative pore-forming subunit. EMBO J. 32, 2362–2376 (2013).
Sancak, Y. et al. EMRE is an essential component of the mitochondrial calcium uniporter complex. Science 342, 1379–1382 (2013). This study used affinity purification of epitope-tagged human MCU followed by quantitative proteomics to characterize the uniporter holocomplex (uniplex), showing that it consists of MCU and its paralogue MCUb, MICU1 and its paralogue MICU2, and a small membrane-spanning, metazoa-specific protein EMRE.
Sather, W. A. & McCleskey, E. W. Permeation and selectivity in calcium channels. Annu. Rev. Physiol. 65, 133–159 (2003).
Chaudhuri, D., Sancak, Y., Mootha, V. K. & Clapham, D. E. MCU encodes the pore conducting mitochondrial calcium currents. eLife 2, e00704 (2013).
Kovacs-Bogdan, E. et al. Reconstitution of the mitochondrial calcium uniporter in yeast. Proc. Natl Acad. Sci. USA 111, 8985–8990 (2014). This study used heterologous expression of human MCU and human EMRE in yeast to show the minimal genetic components that are required for in vivo reconstitution of uniporter activity.
Bragadin, M., Pozzan, T. & Azzone, G. F. Kinetics of Ca2+ carrier in rat liver mitochondria. Biochemistry 18, 5972–5978 (1979).
Igbavboa, U. & Pfeiffer, D. R. EGTA inhibits reverse uniport-dependent Ca2+ release from uncoupled mitochondria. Possible regulation of the Ca2+ uniporter by a Ca2+ binding site on the cytoplasmic side of the inner membrane. J. Biol. Chem. 263, 1405–1412 (1988).
Moreau, B., Nelson, C. & Parekh, A. B. Biphasic regulation of mitochondrial Ca2+ uptake by cytosolic Ca2+ concentration. Curr. Biol. 16, 1672–1677 (2006).
Csordas, G. et al. MICU1 controls both the threshold and cooperative activation of the mitochondrial Ca2+ uniporter. Cell Metab. 17, 976–987 (2013).
Hung, V. et al. Proteomic mapping of the human mitochondrial intermembrane space in live cells via ratiometric APEX tagging. Mol. Cell 55, 332–341 (2014).
Lam, S. S. et al. Directed evolution of APEX2 for electron microscopy and proximity labeling. Nat. Methods 12, 51–54 (2015).
Mallilankaraman, K. et al. MICU1 is an essential gatekeeper for MCU-mediated mitochondrial Ca2+ uptake that regulates cell survival. Cell 151, 630–644 (2012).
Kamer, K. J. & Mootha, V. K. MICU1 and MICU2 play nonredundant roles in the regulation of the mitochondrial calcium uniporter. EMBO Rep. 15, 299–307 (2014).
Kamer, K. J., Sancak, Y. & Mootha, V. K. The uniporter: from newly identified parts to function. Biochem. Biophys. Res. Commun. 449, 370–372 (2014).
Gaj, T., Gersbach, C. A. & Barbas, C. F. 3rd. ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering. Trends Biotechnol. 31, 397–405 (2013).
Hsu, P. D., Lander, E. S. & Zhang, F. Development and applications of CRISPR-Cas9 for genome engineering. Cell 157, 1262–1278 (2014).
Patron, M. et al. MICU1 and MICU2 finely tune the mitochondrial Ca2+ uniporter by exerting opposite effects on MCU activity. Mol. Cell 53, 726–737 (2014).
Wang, L. et al. Structural and mechanistic insights into MICU1 regulation of mitochondrial calcium uptake. EMBO J. 33, 594–604 (2014).
Bygrave, F. L. & Ash, G. R. Development of mitochondrial calcium transport activity in rat liver. FEBS Lett. 80, 271–274 (1977).
Fieni, F., Lee, S. B., Jan, Y. N. & Kirichok, Y. Activity of the mitochondrial calcium uniporter varies greatly between tissues. Nat. Commun. 3, 1317 (2012).
Hoffman, N. E. et al. SLC25A23 augments mitochondrial Ca2+ uptake, interacts with MCU, and induces oxidative stress-mediated cell death. Mol. Biol. Cell 25, 936–947 (2014).
Mallilankaraman, K. et al. MCUR1 is an essential component of mitochondrial Ca2+ uptake that regulates cellular metabolism. Nat. Cell Biol. 14, 1336–1343 (2012).
Paupe, V., Prudent, J., Dassa, E. P., Rendon, O. Z. & Shoubridge, E. A. CCDC90A (MCUR1) is a cytochrome c oxidase assembly factor and not a regulator of the mitochondrial calcium uniporter. Cell Metab. 21, 109–116 (2015).
Palty, R. et al. Lithium-calcium exchange is mediated by a distinct potassium-independent sodium-calcium exchanger. J. Biol. Chem. 279, 25234–25240 (2004).
Trenker, M., Malli, R., Fertschai, I., Levak-Frank, S. & Graier, W. F. Uncoupling proteins 2 and 3 are fundamental for mitochondrial Ca2+ uniport. Nat. Cell Biol. 9, 445–452 (2007).
Shanmughapriya, S. et al. Ca2+ signals regulate mitochondrial metabolism by stimulating CREB-mediated expression of the mitochondrial Ca2+ uniporter gene MCU. Sci. Signal. 8, ra23 (2015).
Qiu, J. et al. Mitochondrial calcium uniporter Mcu controls excitotoxicity and is transcriptionally repressed by neuroprotective nuclear calcium signals. Nat. Commun. 4, 2034 (2013).
Marchi, S. et al. Downregulation of the mitochondrial calcium uniporter by cancer-related miR-25. Curr. Biol. 23, 58–63 (2013).
Pan, X. et al. The physiological role of mitochondrial calcium revealed by mice lacking the mitochondrial calcium uniporter. Nat. Cell Biol. 15, 1464–1472 (2013). This is a report of the surprising finding that genetic ablation of MCU can be tolerated in mice, with the primary phenotype being small size and exercise intolerance.
Alam, M. R. et al. Mitochondrial Ca2+ uptake 1 (MICU1) and mitochondrial Ca2+ uniporter (MCU) contribute to metabolism-secretion coupling in clonal pancreatic β-cells. J. Biol. Chem. 287, 34445–34454 (2012).
Tarasov, A. I. et al. Frequency-dependent mitochondrial Ca2+ accumulation regulates ATP synthesis in pancreatic β cells. Pflugers Arch. 465, 543–554 (2013).
Samanta, K., Douglas, S. & Parekh, A. B. Mitochondrial calcium uniporter MCU supports cytoplasmic Ca2+ oscillations, store-operated Ca2+ entry and Ca2+-dependent gene expression in response to receptor stimulation. PLoS ONE 9, e101188 (2014).
Deak, A. T. et al. IP3-mediated STIM1 oligomerization requires intact mitochondrial Ca2+ uptake. J. Cell Sci. 127, 2944–2955 (2014).
Triantafilou, K., Hughes, T. R., Triantafilou, M. & Morgan, B. P. The complement membrane attack complex triggers intracellular Ca2+ fluxes leading to NLRP3 inflammasome activation. J. Cell Sci. 126, 2903–2913 (2013).
Rimessi, A. et al. Mitochondrial Ca2+-dependent NLRP3 activation exacerbates the Pseudomonas aeruginosa-driven inflammatory response in cystic fibrosis. Nat. Commun. 6, 6201 (2015).
Huang, G., Vercesi, A. E. & Docampo, R. Essential regulation of cell bioenergetics in Trypanosoma brucei by the mitochondrial calcium uniporter. Nat. Commun. 4, 2865 (2013).
Hall, D. D., Wu, Y., Domann, F. E., Spitz, D. R. & Anderson, M. E. Mitochondrial calcium uniporter activity is dispensable for MDA-MB-231 breast carcinoma cell survival. PLoS ONE 9, e96866 (2014).
Xu, S. & Chisholm, A. D. C. elegans epidermal wounding induces a mitochondrial ROS burst that promotes wound repair. Dev. Cell 31, 48–60 (2014).
Murphy, E. et al. Unresolved questions from the analysis of mice lacking MCU expression. Biochem. Biophys. Res. Commun. 449, 384–385 (2014).
Wu, Y. et al. The mitochondrial uniporter controls fight or flight heart rate increases. Nat. Commun. 6, 6081 (2015).
Logan, C. V. et al. Loss-of-function mutations in MICU1 cause a brain and muscle disorder linked to primary alterations in mitochondrial calcium signaling. Nat. Genet. 46, 188–193 (2013). This is the first report of patients with genetic defects of the uniporter, showing that inherited mutations in MICU1 can underlie neuromuscular disease.
Vafai, S. B. & Mootha, V. K. Mitochondrial disorders as windows into an ancient organelle. Nature 491, 374–383 (2012).
Bick, A. G., Calvo, S. E. & Mootha, V. K. Evolutionary diversity of the mitochondrial calcium uniporter. Science 336, 886–886 (2012).
Prudent, J. et al. Bcl-wav and the mitochondrial calcium uniporter drive gastrula morphogenesis in zebrafish. Nat. Commun. 4, 2330 (2013).
van Deursen, J. et al. Skeletal muscles of mice deficient in muscle creatine kinase lack burst activity. Cell 74, 621–631 (1993).
Garry, D. J., Meeson, A., Yan, Z. & Williams, R. S. Life without myoglobin. Cell. Mol. Life Sci. 57, 896–898 (2000).
Yoon, M.J. et al. Release of Ca2+ from the endoplasmic reticulum and its subsequent influx into mitochondria trigger celastrol-induced paraptosis in cancer cells. Oncotarget 5, 6816–6831 (2014).
Wiel, C. et al. Endoplasmic reticulum calcium release through ITPR2 channels leads to mitochondrial calcium accumulation and senescence. Nat. Commun. 5, 3792 (2014).
Hoffman, N.E. et al. MICU1 motifs define mitochondrial calcium uniporter binding and activity. Cell Rep. 5, 1576–1588 (2013).
Drago, I., De Stefani, D., Rizzuto, R. & Pozzan, T. Mitochondrial Ca2+ uptake contributes to buffering cytoplasmic Ca2+ peaks in cardiomyocytes. Proc. Natl Acad. Sci. USA 109, 12986–12991 (2012).
Mammucari, C. et al. The mitochondrial calcium uniporter controls skeletal muscle trophism in vivo. Cell Rep. 10, 1269–1279 (2015).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Mitoplast
-
A mitochondrion without the outer membrane.
- EF hand
-
A calcium-binding motif consisting of a helix–loop–helix structure.
- Excitation–energetic coupling
-
The coupling of cellular ATP consumption with its production.
- Leukotriene receptor
-
A receptor that is present in immune cells, which can be activated by leukotrienes to lead to an inflammatory cascade.
- Store-operated calcium entry
-
(SOCE). A mechanism to replenish endoplasmic reticulum calcium stores, which occurs through calcium release-activated channels in the plasma membrane.
- NLRP3 inflammasome
-
A large protein complex that is part of the innate immune system; it can be activated by many different stimuli to trigger inflammatory processes.
- Tricarboxylic acid (TCA) cycle
-
A series of enzymatic reactions in the mitochondrial matrix that take acetyl coenzyme A through a series of oxidation steps, which are important for many biosynthetic pathways and also produce reducing equivalents to feed into the respiratory chain. Two enzymes in the TCA cycle, α-ketoglutarate dehydrogenase and isocitrate dehydrogenase, are activated by matrix calcium ions.
- Skeletal muscle myopathy
-
A disorder of skeletal muscle that can manifest as weakness, cramps or exercise intolerance.
Rights and permissions
About this article
Cite this article
Kamer, K., Mootha, V. The molecular era of the mitochondrial calcium uniporter. Nat Rev Mol Cell Biol 16, 545–553 (2015). https://doi.org/10.1038/nrm4039
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrm4039
This article is cited by
-
Decreasing mitochondrial fission ameliorates HIF-1α-dependent pathological retinal angiogenesis
Acta Pharmacologica Sinica (2024)
-
Reduced mitochondrial calcium uptake in macrophages is a major driver of inflammaging
Nature Aging (2023)
-
Deficiency of mitochondrial calcium uniporter abrogates iron overload-induced cardiac dysfunction by reducing ferroptosis
Basic Research in Cardiology (2023)
-
The status and trends of mitochondrial dynamics research: A global bibliometric and visualized analysis
Journal of Bioenergetics and Biomembranes (2023)
-
MiR-25 overexpression inhibits titanium particle-induced osteoclast differentiation via down-regulation of mitochondrial calcium uniporter in vitro
Journal of Orthopaedic Surgery and Research (2022)