Abstract
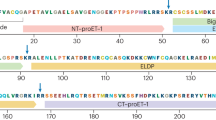
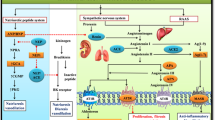
Cardiovascular disease is a major contributor to global morbidity and mortality and is the common end point of many chronic diseases. The endothelins comprise three structurally similar peptides of 21 amino acids in length. Endothelin 1 (ET-1) and ET-2 activate two G protein-coupled receptors — endothelin receptor type A (ETA) and endothelin receptor type B (ETB) — with equal affinity, whereas ET-3 has a lower affinity for ETA. ET-1 is the most potent vasoconstrictor in the human cardiovascular system and has remarkably long-lasting actions. ET-1 contributes to vasoconstriction, vascular and cardiac hypertrophy, inflammation, and to the development and progression of cardiovascular disease. Endothelin receptor antagonists have revolutionized the treatment of pulmonary arterial hypertension. Clinical trials continue to explore new applications of endothelin receptor antagonists, particularly in treatment-resistant hypertension, chronic kidney disease and patients receiving antiangiogenic therapies. Translational studies have identified important roles for the endothelin isoforms and new therapeutic targets during development, in fluid-electrolyte homeostasis, and in cardiovascular and neuronal function. Novel pharmacological strategies are emerging in the form of small-molecule epigenetic modulators, biologics (such as monoclonal antibodies for ETB) and possibly signalling pathway-biased agonists and antagonists.
Key points
-
Endothelin 1 (ET-1) is the most potent endogenous vasoconstrictor and contributes to basal vascular tone as well as a number of diseases, such as hypertension, chronic kidney disease (CKD), pulmonary arterial hypertension (PAH) and pre-eclampsia.
-
A common non-coding gene sequence variant regulates the expression of EDN1 (encoding ET-1) and is linked to five common vascular conditions: coronary artery disease, hypertension, migraine, cervical artery dissection and fibromuscular hyperplasia.
-
The effects of ET-1 are mediated via two G protein-coupled receptors, endothelin receptor type A (ETA) and endothelin receptor type B (ETB); selective ETA and dual ETA/ETB receptor antagonists (endothelin receptor antagonists; ERAs) are available for clinical use.
-
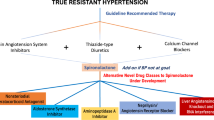
The phase III PRECISION study will explore the use of a long-acting, selective ETA antagonist in treatment-resistant hypertension.
-
For many patients with PAH, ERAs are considered first-line treatment; ERAs also reduce blood pressure and proteinuria in CKD and show clinical potential in scleroderma renal crisis, the transition from acute kidney injury to CKD and end-stage renal disease.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Hickey, K. A., Rubanyi, G., Paul, R. J. & Highsmith, R. F. Characterization of a coronary vasoconstrictor produced by cultured endothelial cells. Am. J. Physiol. 248, C550–C556 (1985).
Yanagisawa, M. et al. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature 332, 411–415 (1988).
Haynes, W. G. & Webb, D. J. Contribution of endogenous generation of endothelin-1 to basal vascular tone. Lancet 344, 852–854 (1994).
Haynes, W. G. et al. Systemic endothelin receptor blockade decreases peripheral vascular resistance and blood pressure in humans. Circulation 93, 1860–1870 (1996).
MacIntyre, I. M. et al. Greater functional ETB receptor antagonism with bosentan than sitaxsentan in healthy men. Hypertension 55, 1406–1411 (2010).
Davenport, P. et al. Endothelin. Pharmacol. Rev. 68, 357–418 (2016).
Ge, Y. et al. Collecting duct-specific knockout of the endothelin B receptor causes hypertension and sodium retention. Am. J. Physiol. 291, F1274–F1280 (2006).
Gupta, R. M. et al. A genetic variant associated with five vascular diseases is a distal regulator of endothelin-1 gene expression. Cell 170, 522–533 (2017).
Boss, C., Bolli, M. H. & Gatfield, J. From bosentan (Tracleer(R)) to macitentan (Opsumit(R)): The medicinal chemistry perspective. Bioorg. Med. Chem. Lett. 26, 3381–3394 (2016).
Wei, A. et al. Clinical adverse effects of endothelin receptor antagonists: insights from the meta-analysis of 4894 patients from 24 randomized double-blind placebo-controlled clinical trials. J. Am. Heart Assoc. 5, e003896 (2016).
Lariviere, R., Day, R. & Schiffrin, E. L. Increased expression of endothelin-1 gene in blood vessels of deoxycorticosterone acetate-salt hypertensive rats. Hypertension 21, 916–920 (1993).
Li, J. S., Lariviere, R. & Schiffrin, E. L. Effect of a nonselective endothelin antagonist on vascular remodeling in deoxycorticosterone acetate-salt hypertensive rats. Evidence for a role of endothelin in vascular hypertrophy. Hypertension 24, 183–188 (1994).
GBD 2016 Risk Factors Collaborators. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 390, 1345–1422 (2017).
Krum, H., Viskoper, R. J., Lacourciere, Y., Budde, M. & Charlon, V. The effect of an endothelin-receptor antagonist, bosentan, on blood pressure in patients with essential hypertension. N. Engl. J. Med. 338, 784–790 (1998).
Nakov, R., Pfarr, E. & Eberle, S. Darusentan: an effective endothelinA receptor antagonist for treatment of hypertension. Am. J. Hypertens. 15, 583–589 (2002).
Calhoun, D. A. et al. Resistant hypertension: diagnosis, evaluation, and treatment. A scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension 51, 1403–1419 (2008).
Schiffrin, E. L., Deng, L. Y., Sventek, P. & Day, R. Enhanced expression of endothelin-1 gene in resistance arteries in severe human essential hypertension. J. Hypertens. 15, 57–63 (1997).
Weber, M. A. et al. A selective endothelin-receptor antagonist to reduce blood pressure in patients with treatment-resistant hypertension: a randomised, double-blind, placebo-controlled trial. Lancet 374, 1423–1431 (2009).
Bakris, G. L. et al. Divergent results using clinic and ambulatory blood pressures: report of a darusentan-resistant hypertension trial. Hypertension 56, 824–830 (2010).
Webb, D. J. DORADO: opportunity postponed: lessons from studies of endothelin receptor antagonists in treatment-resistant hypertension. Hypertension 56, 806–807 (2010).
Williams, B. et al. Spironolactone versus placebo, bisoprolol, and doxazosin to determine the optimal treatment for drug-resistant hypertension (PATHWAY-2): a randomised, double-blind, crossover trial. Lancet 386, 2059–2068 (2015).
Iglarz, M. et al. Pharmacology of macitentan, an orally active tissue-targeting dual endothelin receptor antagonist. J. Pharmacol. Exp. Ther. 327, 736–745 (2008).
Idorsia. Drug discovery & clinical development. idorsia https://www.idorsia.com/documents/com/fact-sheets-presentations/fs-clinical-development.pdf (2019).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02603809 (2019).
Nelson, J. B. et al. Phase 3, randomized, placebo-controlled study of zibotentan (ZD4054) in patients with castration-resistant prostate cancer metastatic to bone. Cancer 118, 5709–5718 (2012).
Vercauteren, M. et al. Endothelin ETA receptor blockade, by activating ETB receptors, increases vascular permeability and induces exaggerated fluid retention. J. Pharmacol. Exp. Ther. 361, 322–333 (2017).
Sakai, S. et al. Endogenous endothelin-1 participates in the maintenance of cardiac function in rats with congestive heart failure. Marked increase in endothelin-1 production in the failing heart. Circulation 93, 1214–1222 (1996).
Sakai, S. et al. Inhibition of myocardial endothelin pathway improves long-term survival in heart failure. Nature 384, 353–355 (1996).
Gray, G. A. & Webb, D. J. The endothelin system and its potential as a therapeutic target in cardiovascular disease. Pharmacol. Ther. 72, 109–148 (1996).
Kiowski, W. et al. Evidence for endothelin-1-mediated vasoconstriction in severe chronic heart failure. Lancet 346, 732–736 (1995).
Cowburn, P. J. et al. Short-term haemodynamic effects of BQ-123, a selective endothelin ETA-receptor antagonist, in chronic heart failure. Lancet 352, 201–202 (1998).
Kelland, N. F. & Webb, D. J. Clinical trials of endothelin antagonists in heart failure: publication is good for the public health. Heart 93, 2–4 (2007).
Battistini, B., Berthiaume, N., Kelland, N. F., Webb, D. J. & Kohan, D. E. Profile of past and current clinical trials involving endothelin receptor antagonists: the novel “-sentan” class of drugs. Exp. Biol. Med. (Maywood) 231, 653–695 (2006).
Anand, I. et al. Long-term effects of darusentan on left-ventricular remodelling and clinical outcomes in the Endothelin A Receptor Antagonist Trial in Heart Failure (EARTH): randomised, double-blind, placebo-controlled trial. Lancet 364, 327–354 (2004).
Kaluski, E. et al. Clinical and hemodynamic effects of bosentan dose optimization in symptomatic heart failure patients with severe systolic dysfunction, associated with secondary pulmonary hypertension – a multi-center randomized study. Cardiology 109, 273–280 (2008).
Zile, M. R. et al. Randomized, double-blind, placebo-controlled study of sitaxsentan to improve impaired exercise tolerance in patients with heart failure and a preserved ejection fraction. JACC Heart Fail. 2, 123–130 (2014).
Teerlink, J. R. in Acute Heart Failure (eds Mebazaa, A., Gheorghiade, M., Zannad, F. M. & Parrillo, J. E.) 626–638 (Springer, 2008).
O’Connor, C. M. et al. Tezosentan in patients with acute heart failure and acute coronary syndromes: results of the Randomized Intravenous TeZosentan Study (RITZ-4). J. Am. Coll. Cardiol. 41, 1452–1457 (2003).
Kaluski, E. et al. RITZ-5: randomized intravenous TeZosentan (an endothelin-A/B antagonist) for the treatment of pulmonary edema: a prospective, multicenter, double-blind, placebo-controlled study. J. Am. Coll. Cardiol. 41, 204–210 (2003).
McMurray, J. J. et al. Effects of tezosentan on symptoms and clinical outcomes in patients with acute heart failure: the VERITAS randomized controlled trials. JAMA 298, 2009–2019 (2007).
Vaduganathan, M., Greene, S. J., Ambrosy, A. P., Gheorghiade, M. & Butler, J. The disconnect between phase II and phase III trials of drugs for heart failure. Nat. Rev. Cardiol. 10, 85–97 (2013).
Kelland, N. F. & Webb, D. J. Clinical trials of endothelin antagonists in heart failure: a question of dose? Exp. Biol. Med. (Maywood) 231, 696–699 (2006).
D’Alonzo, G. E. et al. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann. Intern. Med. 115, 343–349 (1991).
Motte, S., McEntee, K. & Naeije, R. Endothelin receptor antagonists. Pharmacol. Ther. 110, 386–414 (2006).
Dupuis, J., Stewart, D. J., Cernacek, P. & Gosselin, G. Human pulmonary circulation is an important site for both clearance and production of endothelin-1. Circulation 94, 1278–1284 (1996).
Dupuis, J., Moe, G. W. & Cernacek, P. Reduced pulmonary metabolism of endothelin-1 in canine tachycardia-induced heart failure. Cardiovasc. Res. 39, 609–616 (1998).
Rubin, L. J. et al. Bosentan therapy for pulmonary arterial hypertension. N. Engl. J. Med. 346, 896–903 (2002).
Ivy, D. et al. Endothelin B receptor deficiency potentiates ET-1 and hypoxic pulmonary vasoconstriction. Am. J. Physiol. 280, L1040–L1048 (2001).
Muramatsu, M. et al. Chronic hypoxia augments endothelin-B receptor-mediated vasodilation in isolated perfused rat lungs. Am. J. Physiol. 276, L358–L364 (1999).
Ivy, D. D., Parker, T. A. & Abman, S. H. Prolonged endothelin B receptor blockade causes pulmonary hypertension in the ovine fetus. Am. J. Physiol. 279, L758–L765 (2000).
Eddahibi, S., Raffestin, B., Clozel, M., Levame, M. & Adnot, S. Protection from pulmonary hypertension with an orally active endothelin receptor antagonist in hypoxic rats. Am. J. Physiol. 268, H828–H835 (1995).
Sato, K. et al. Effects of separate and combined ETA and ETB blockade on ET-1-induced constriction in perfused rat lungs. Am. J. Physiol. 269, L668–L672 (1995).
Galie, N. et al. Initial use of ambrisentan plus tadalafil in pulmonary arterial hypertension. N. Engl. J. Med. 373, 834–844 (2015).
Thenappan, T., Ormiston, M. L., Ryan, J. J. & Archer, S. L. Pulmonary arterial hypertension: pathogenesis and clinical management. BMJ 360, j5492 (2018).
Galie, N. et al. Treatment of patients with mildly symptomatic pulmonary arterial hypertension with bosentan (EARLY study): a double-blind, randomised controlled trial. Lancet 371, 2093–2100 (2008).
Pulido, T. et al. Macitentan and morbidity and mortality in pulmonary arterial hypertension. N. Engl. J. Med. 369, 809–818 (2013).
Galie, N. et al. Ambrisentan for the treatment of pulmonary arterial hypertension: results of the ambrisentan in pulmonary arterial hypertension, randomized, double-blind, placebo-controlled, multicenter, efficacy (ARIES) study 1 and 2. Circulation 117, 3010–3019 (2008).
Hocher, B. et al. Pulmonary fibrosis and chronic lung inflammation in ET-1 transgenic mice. Am. J. Respir. Cell Mol. Biol. 23, 19–26 (2000).
Davie, N. et al. ET(A) and ET(B) receptors modulate the proliferation of human pulmonary artery smooth muscle cells. Am. J. Respir. Crit. Care Med. 165, 398–405 (2002).
Nishida, M. et al. Role of endothelin ETB receptor in the pathogenesis of monocrotaline-induced pulmonary hypertension in rats. Eur. J. Pharmacol. 496, 159–165 (2004).
Nishida, M., Eshiro, K., Okada, Y., Takaoka, M. & Matsumura, Y. Roles of endothelin ETA and ETB receptors in the pathogenesis of monocrotaline-induced pulmonary hypertension. J. Cardiovasc. Pharmacol. 44, 187–191 (2004).
de Zeeuw, D. et al. Proteinuria, a target for renoprotection in patients with type 2 diabetic nephropathy: lessons from RENAAL. Kidney Int. 65, 2309–2320 (2004).
Dhaun, N. et al. Blood pressure-independent reduction in proteinuria and arterial stiffness after acute endothelin-a receptor antagonism in chronic kidney disease. Hypertension 54, 113–119 (2009).
Dhaun, N. et al. Selective endothelin-A receptor antagonism reduces proteinuria, blood pressure, and arterial stiffness in chronic proteinuric kidney disease. Hypertension 57, 772–779 (2011).
Dhaun, N. et al. Endothelin-A receptor antagonism modifies cardiovascular risk factors in CKD. J. Am. Soc. Nephrol. 24, 31–36 (2013).
Wenzel, R. R. et al. Avosentan reduces albumin excretion in diabetics with macroalbuminuria. J. Am. Soc. Nephrol. 60, 655–664 (2009).
Kohan, D. E. et al. Addition of atrasentan to renin-angiotensin system blockade reduces albuminuria in diabetic nephropathy. J. Am. Soc. Nephrol. 22, 763–772 (2011).
Dhaun, N. et al. Haemodynamic and renal effects of endothelin receptor antagonism in patients with chronic kidney disease. Nephrol. Dial. Transplant. 22, 3228–3234 (2007).
de Zeeuw, D. et al. The endothelin antagonist atrasentan lowers residual albuminuria in patients with type 2 diabetic nephropathy. J. Am. Soc. Nephrol. 25, 1083–1093 (2014).
Czopek, A., Moorhouse, R., Webb, D. J. & Dhaun, N. Therapeutic potential of endothelin receptor antagonism in kidney disease. Am. J. Physiol. 310, R388–R397 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01858532 (2018).
Neuen, B. L. et al. Cardiovascular and renal outcomes with canagliflozin according to baseline kidney function. Circulation 138, 1537–1550 (2018).
Trachtman, H. et al. DUET: a phase 2 study evaluating the efficacy and safety of sparsentan in patients with FSGS. J. Am. Soc. Nephrol. 29, 2745–2754 (2018).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03493685 (2018).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03762850 (2019).
Parvanova, A. et al. Effect on blood pressure of combined inhibition of endothelin-converting enzyme and neutral endopeptidase with daglutril in patients with type 2 diabetes who have albuminuria: a randomised, crossover, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 1, 19–27 (2013).
Ferro, C. J., Spratt, J. C., Haynes, W. G. & Webb, D. J. Inhibition of neutral endopeptidase causes vasoconstriction of human resistance vessels in vivo. Circulation 97, 2323–2330 (1998).
Hsu, C. Y. Where is the epidemic in kidney disease? J. Am. Soc. Nephrol. 21, 1607–1611 (2010).
Lameire, N. H. et al. Acute kidney injury: an increasing global concern. Lancet 382, 170–179 (2013).
Odutayo, A. et al. AKI and long-term risk for cardiovascular events and mortality. J. Am. Soc. Nephrol. 28, 377–387 (2017).
Wald, R. et al. Chronic dialysis and death among survivors of acute kidney injury requiring dialysis. JAMA 302, 1179–1185 (2009).
Dhaun, N., Goddard, J. & Webb, D. J. The endothelin system and its antagonism in chronic kidney disease. J. Am. Soc. Nephrol. 17, 943–955 (2006).
Wilhelm, S. M., Simonson, M. S., Robinson, A. V., Stowe, N. T. & Schulak, J. A. Endothelin up-regulation and localization following renal ischemia and reperfusion. Kidney Int. 55, 1011–1018 (1999).
Zager, R. A., Johnson, A. C., Andress, D. & Becker, K. Progressive endothelin-1 gene activation initiates chronic/end-stage renal disease following experimental ischemic/reperfusion injury. Kidney Int. 84, 703–712 (2013).
Gellai, M., Jugus, M., Fletcher, T., DeWolf, R. & Nambi, P. Reversal of postischemic acute renal failure with a selective endothelin A receptor antagonist in the rat. J. Clin. Invest. 93, 900–906 (1994).
Huang, C. et al. The effect of endothelin antagonists on renal ischaemia-reperfusion injury and the development of acute renal failure in the rat. Nephrol. Dial. Transplant. 17, 1578–1585 (2002).
Forbes, J. M., Leaker, B., Hewitson, T. D., Becker, G. J. & Jones, C. L. Macrophage and myofibroblast involvement in ischemic acute renal failure is attenuated by endothelin receptor antagonists. Kidney Int. 55, 198–208 (1999).
Barrera-Chimal, J. et al. Sulfenic acid modification of endothelin B receptor is responsible for the benefit of a nonsteroidal mineralocorticoid receptor antagonist in renal ischemia. J. Am. Soc. Nephrol. 27, 398–404 (2016).
de Jager, D. J. et al. Cardiovascular and noncardiovascular mortality among patients starting dialysis. JAMA 302, 1782–1789 (2009).
Totsune, K. et al. Detection of immunoreactive endothelin in plasma of hemodialysis patients. FEBS Lett. 249, 239–242 (1989).
Lariviere, R. et al. Endothelin type A receptor blockade reduces vascular calcification and inflammation in rats with chronic kidney disease. J. Hypertens. 35, 376–384 (2017).
Briet, M., Boutouyrie, P., Laurent, S. & London, G. M. Arterial stiffness and pulse pressure in CKD and ESRD. Kidney Int. 82, 388–400 (2012).
Fuquay, R. et al. Renal ischemia-reperfusion injury amplifies the humoral immune response. J. Am. Soc. Nephrol. 24, 1063–1072 (2013).
Raina, A., Horn, E. T. & Benza, R. L. The pathophysiology of endothelin in complications after solid organ transplantation: a potential novel therapeutic role for endothelin receptor antagonists. Transplantation 94, 885–893 (2012).
Penn, H. et al. Scleroderma renal crisis: patient characteristics and long-term outcomes. QJM 100, 485–494 (2007).
Denton, C. P. Renal manifestations of systemic sclerosis — clinical features and outcome assessment. Rheumatology 47 (Suppl. 5), v54–v56 (2008).
Abraham, D. J. et al. Increased levels of endothelin-1 and differential endothelin type A and B receptor expression in scleroderma-associated fibrotic lung disease. Am. J. Pathol. 151, 831–841 (1997).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02047708 (2017).
Morris, C. D. et al. Specific inhibition of the endothelin A receptor with ZD4054: clinical and pre-clinical evidence. Br. J. Cancer 92, 2148–2152 (2005).
Andresen, J., Shafi, N. I. & Bryan, R. M. Jr. Endothelial influences on cerebrovascular tone. J. Appl. Physiol. 100, 318–327 (2006).
Matsuo, Y., Mihara, S., Ninomiya, M. & Fujimoto, M. Protective effect of endothelin type A receptor antagonist on brain edema and injury after transient middle cerebral artery occlusion in rats. Stroke 32, 2143–2148 (2001).
Petrov, T. & Rafols, J. A. Acute alterations of endothelin-1 and iNOS expression and control of the brain microcirculation after head trauma. Neurol. Res. 23, 139–143 (2001).
Chow, M., Dumont, A. S. & Kassell, N. F. Endothelin receptor antagonists and cerebral vasospasm: an update. Neurosurgery 51, 1333–1341 (2002).
Macdonald, R. L., Pluta, R. M. & Zhang, J. H. Cerebral vasospasm after subarachnoid hemorrhage: the emerging revolution. Nat. Clin. Pract. Neurol. 3, 256–263 (2007).
Vergouwen, M. D., Algra, A. & Rinkel, G. J. Endothelin receptor antagonists for aneurysmal subarachnoid hemorrhage: a systematic review and meta-analysis update. Stroke 43, 2671–2676 (2012).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02560532 (2018).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03585270 (2019).
Saleh, L., Danser, J. A. & van den Meiracker, A. H. Role of endothelin in preeclampsia and hypertension following antiangiogenesis treatment. Curr. Opin. Nephrol. Hypertens. 25, 94–99 (2016).
Lankhorst, S., Kappers, M. H., van Esch, J. H., Danser, A. H. & van den Meiracker, A. H. Mechanism of hypertension and proteinuria during angiogenesis inhibition: evolving role of endothelin-1. J. Hypertens. 31, 444–454 (2013).
Eremina, V. et al. VEGF inhibition and renal thrombotic microangiopathy. N. Engl. J. Med. 358, 1129–1136 (2008).
Kappers, M. H. et al. Hypertension induced by the tyrosine kinase inhibitor sunitinib is associated with increased circulating endothelin-1 levels. Hypertension 56, 675–681 (2010).
de Jesus-Gonzalez, N. et al. Regorafenib induces rapid and reversible changes in plasma nitric oxide and endothelin-1. Am. J. Hypertens. 25, 1118–1123 (2012).
Verdonk, K. et al. Association studies suggest a key role for endothelin-1 in the pathogenesis of preeclampsia and the accompanying renin-angiotensin-aldosterone system suppression. Hypertension 65, 1316–1323 (2015).
Zhou, J. et al. Gestational hypoxia induces preeclampsia-like symptoms via heightened endothelin-1 signaling in pregnant rats. Hypertension 62, 599–607 (2013).
Murphy, S. R., LaMarca, B. B., Cockrell, K. & Granger, J. P. Role of endothelin in mediating soluble fms-like tyrosine kinase 1-induced hypertension in pregnant rats. Hypertension 55, 394–398 (2010).
Alexander, B. T. et al. Endothelin type A receptor blockade attenuates the hypertension in response to chronic reductions in uterine perfusion pressure. Hypertension 37, 485–489 (2001).
Lankhorst, S. et al. Treatment of hypertension and renal injury induced by the angiogenesis inhibitor sunitinib: preclinical study. Hypertension 64, 1282–1289 (2014).
Kappers, M. H. et al. Sunitinib-induced systemic vasoconstriction in swine is endothelin mediated and does not involve nitric oxide or oxidative stress. Hypertension 59, 151–157 (2012).
Rosano, L., Spinella, F. & Bagnato, A. Endothelin 1 in cancer: biological implications and therapeutic opportunities. Nat. Rev. Cancer 13, 637–651 (2013).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT03557190 (2018).
Bushnell, C. et al. Guidelines for the prevention of stroke in women: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 45, 1545–1588 (2014).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT02712346 (2019).
Acknowledgements
N.D. has research funding from the Medical Research Council to explore the role of the endothelin system in kidney disease (MRC ref: 6152177 I181211-0759).
Reviewer information
Nature Reviews Cardiology thanks E. Schiffrin and the other anonymous reviewer(s), for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
Both authors researched data for the article, discussed its content and wrote the manuscript. D.J.W. reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
N.D. has acted as a consultant for Retrophin. D.J.W. is a member of an Independent Data Monitoring Committee for a trial with AbbVie of atrasentan in diabetic renal disease and is the UK Chief Investigator for a trial with Idorsia of aprocitentan in treatment-resistant hypertension.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dhaun, N., Webb, D.J. Endothelins in cardiovascular biology and therapeutics. Nat Rev Cardiol 16, 491–502 (2019). https://doi.org/10.1038/s41569-019-0176-3
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41569-019-0176-3
This article is cited by
-
Resistant Hypertension: Disease Burden and Emerging Treatment Options
Current Hypertension Reports (2024)
-
Current and future strategies for targeting the endothelin pathway in cardiovascular disease
Nature Cardiovascular Research (2023)
-
Hypoxia-induced signaling in the cardiovascular system: pathogenesis and therapeutic targets
Signal Transduction and Targeted Therapy (2023)
-
Endothelin-1 is associated with mortality that can be attenuated with high intensity statin therapy in patients with stable coronary artery disease
Communications Medicine (2023)
-
Dual Endothelin Antagonism with Aprocitentan as a Novel Therapeutic Approach for Resistant Hypertension
Current Hypertension Reports (2023)