-
PDF
- Split View
-
Views
-
Cite
Cite
M. Al-Asmakh, H. Race, S. Tan, M.H.F. Sullivan, The effects of oxygen concentration on in vitro output of prostaglandin E2 and interleukin-6 from human fetal membranes, Molecular Human Reproduction, Volume 13, Issue 3, March 2007, Pages 197–201*, https://doi.org/10.1093/molehr/gal109
Close - Share Icon Share
Abstract
Labour at all gestational ages has clear biochemical parallels with an inflammatory response, typified by the increased output of prostaglandins (PGs) and cytokines within the pregnant uterus. The main sources are the fetal membranes, including the amnion, chorion and decidua, and it is well established that stimuli [bacteria, bacterial endotoxins, interleukin (IL)-1β, corticotrophin releasing hormone and platelet activating factor], as well as negative regulators (progesterone and IL-10), control the net output of PGs and cytokines in vitro. In this study, we have investigated the effect of oxygen tension on fetal membrane biology, as a reconsideration of the literature suggests that fetal membranes are normally exposed to ∼3% O2 (∼20 mmHg) in vivo, rather than the 20% O2 (150 mmHg) used for in vitro culture. The output of prostaglandin E2 from non-activated fetal membranes in response to IL-1β was decreased by ∼80% at 16 and 24 h of culture, whereas the inhibition of IL-6 production was time-dependent, reaching 90% after 16 h and 50% after 24 h. Tissues obtained after labour (or after the activation of inflammatory processes leading to labour) were not inhibited by the low levels of oxygen, indicating that only before the onset of labour does oxygen regulate fetal membrane biology. The data identify oxygen as a regulator of fetal membrane inflammatory functions during human pregnancy, and its mechanism of action requires further study.
Introduction
Pathologically, early (preterm) and normal term labour resembles inflammatory processes, as typified by the increased production of prostaglandins (PGs) and cytokines (Kelly, 1996). Prostaglandins E2 (PGE2) is a critical regulator of cervical ripening (Calder and Greer, 1991), whereas PGF2α co-ordinates myometrial contractility (Fuchs and Fuchs, 1984). Interleukin (IL)-8 recruits neutrophils to the cervix and fetal membranes (Kelly et al., 1994), leading to the remodelling of these tissues (ripening and rupture, respectively) via the activity of neutrophil proteases. IL-1β potently up-regulates the output of both PGs, IL-8 and IL-6 (Keelan et al., 2003), and is a key factor in labour. IL-6 can increase PG output (Mitchell et al., 1991), but its other roles are less well understood; it is the best marker of preterm labour and inflammatory changes within the uterus (Romero et al., 1993).
Activation of PG and cytokine production has been most reproducibly detected within fetal membranes, and this applies both to changes during labour (Bowen et al., 2002; Keelan et al., 2003) and in vitro models of these processes (Brown et al., 1998, 2000; Rajasingam et al., 1998). Inhibition of this activation allows the continuation of pregnancy, and anti-inflammatory cytokines and progesterone suppress the inflammatory process in vitro (Kelly et al., 1994; Alvi et al., 1999; Loudon et al., 2003). In the particular case of infection-induced preterm labour (Romero et al., 1991), many different organisms have been linked to preterm labour (Romero et al., 2002). This suggests that the intrauterine inflammation is a generic response to bacteria, rather than the result of specific pathogens, but a recent paper has suggested that Fusobacterium nucleatum (F. nucleatum) may be important in women in preterm labour with prolonged rupture of the fetal membranes (Cahill et al., 2005). Fusobacterium nucleatum is an obligate anaerobe killed by oxygen tensions exceeding 6% (Loesche, 1969), indicating that oxygen levels within some compartments of the term uterus do not exceed this value.
Oxygen is an important regulator of placental function, and it is well established that the placenta develops under relatively hypoxic conditions for the first 8–10 weeks of pregnancy (Jauniaux et al., 2000, 2001), and in vitro studies on first trimester placenta must be performed at appropriate oxygen concentrations (3%) (Miller et al., 2005). All data on term fetal membranes have used atmospheric oxygen (20%) during culture, but three lines of evidence suggest that this may not be appropriate. First, the presence of obligate anaerobes such as F. nucleatum in fetal membranes (Cahill et al., 2005) suggests a low ambient oxygen level, as indicated earlier. Secondly, the amniotic fluid is known to be relatively hypoxic throughout pregnancy (Johnell and Nilsson, 1971), and this will affect the functions of the amnion component of fetal membranes at least. Finally, the chorion has a limited blood supply (and the amnion is totally avascular), suggesting that the oxygen supply to these tissues may be limited.
The aim of this study is to determine whether changes in O2 during culture affect the response of human fetal membranes to IL-1β.
Methods
Fetal membranes were obtained from normal term human pregnancies, delivered either by elective Caesarean section before labour or after spontaneous vaginal delivery. The study was approved by the Hammersmith and Queen Charlotte's Hospitals Research Ethics Committee, and informed written consent was obtained from all patients. After delivery, fetal membranes were collected into sterile phosphate-buffered saline + antibiotics (1% v/v penicillin–streptomycin) for transfer to the laboratory and fetal membrane explants cultured as described previously (Rajasingam et al., 1998), with the exception of the oxygen levels indicated below. All materials used in this study were from Sigma-Aldrich Company Ltd (Gillingham, UK) unless otherwise stated. Some tissues were incubated in serum-free Dulbeco's Modified Eagle's medium at 37°C in 95% air: 5% CO2 (standard conditions), whereas others were in an atmosphere with 3% oxygen: 5% CO2 (relative hypoxia, maintained by provision of N2) from the time that the tissues were placed in culture, such that the normal overnight recovery period was at the oxygen concentration that would be used for the rest of the study. At the end of the incubations, culture media were removed and frozen at − 20°C until assay for PGE2 (GE Healthcare, Amersham, UK) and for IL-6 (Biosource, Nivelles, Belgium). Assays were performed according to the manufacturers' instructions. Preliminary data showed that the effects of IL-1β on the output of PGE2 were decreased by 20% after 24 h of culture at low oxygen levels. Detailed time course studies were then performed, so that explants were cultured for 4, 6, 8, 16 and 24 h under standard or relatively hypoxic conditions ± IL-1β at 1 ng ml−1. PGE2 was assessed only after 16 and 24 h, as the initial experiments showed that the greatest differences were observed at longer time intervals. This also allows identification of ‘non-activated’ and ‘activated’ term fetal membrane tissues; the latter show the increased PGE2 output of labour (Brown et al., 1998), but before labour can be clinically recognized, and the latter tissues do not respond to inflammatory stimuli (Brown et al., 1998). Comparisons between levels of PGE2 and IL-6 were assessed by analysis of variance, and differences of P < 0.05 were considered significant.
Results
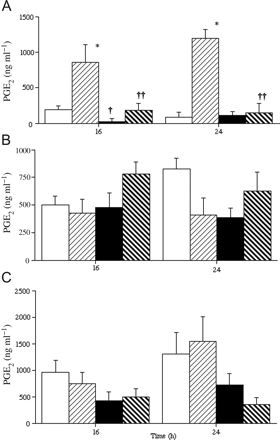
Non-activated tissues produced high levels of PGE2 when stimulated with IL-1β under standard culture conditions after 16 and 24 h (Figure 1A). Low levels of oxygen decreased basal PGE2 output only after 16 h and almost eliminated the stimulatory effect of IL-1β (Figure 1A). Activated and post-labour (Figure 1B and C, respectively) tissues produced high basal levels of PGE2 as expected (Brown et al., 1998) and these were not increased by IL-1β. Culture of the activated or post-labour tissues under relative hypoxia was also without effect (except in post-labour tissues after 24 h) (Figure 1C).
Output of prostaglandin E2 (PGE2) from fetal membranes from elective Caesarean section deliveries (A and B) or after normal term labour (C). Tissues in (A) show a non-activated pattern of PGE2 output (Brown et al., 1998), with low basal output that is increased by interleuki (IL)-1β. Tissues were cultured in 20% (□,  ) or 3% (▪,
) or 3% (▪,  ) oxygen, with (
) oxygen, with ( ,
,  ) or without (□, ▪) IL-1β at a concentration of 1 ng ml−1 for the time periods shown. All data are mean ± SEM (n = 3–5).
) or without (□, ▪) IL-1β at a concentration of 1 ng ml−1 for the time periods shown. All data are mean ± SEM (n = 3–5).
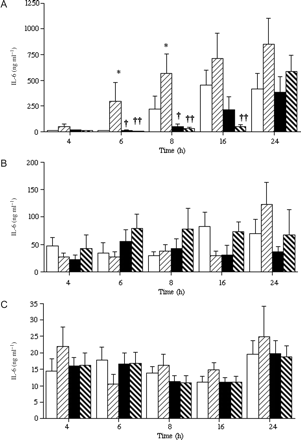
IL-6 production was determined in media from these same tissues (Figure 2). In the non-activated tissues, IL-1β increased IL-6 output significantly after 6–8 h, and there was a trend towards higher IL-6 output after 16–24 h (Figure 2A). Tissues cultured at a low oxygen tension produced lower basal levels of IL-6 (6–8 h), and the effects of IL-1β were decreased (6, 8 and 16 h) (Figure 2A). Activated fetal membranes (identified by high basal PGE2 output, Figure 1) produced lower levels of IL-6 (50–100 ng ml−1), and neither IL-1β nor oxygen tension had any effects on IL-6 output (Figure 2B). A similar lack of effect of oxygen or IL-1β was observed in post-labour tissues (Figure 2C), and overall IL-6 output was generally lower still (15–30 ng ml−1).
Output of interleukin (IL)-6 from fetal membranes from elective Caesarean section deliveries (A and B) or after normal term labour (C). Tissues are grouped as in Figure 1, such that (A) is non-activated term tissues and (B) is activated tissues before labour. Tissues were cultured in 20% (□,  ) or 3% (▪,
) or 3% (▪,  ) oxygen, with (
) oxygen, with ( ,
,  ) or without (□, ▪) IL-1β at a concentration of 1 ng ml−1 for the time periods shown. All data are mean ± SEM (n = 3–5).
) or without (□, ▪) IL-1β at a concentration of 1 ng ml−1 for the time periods shown. All data are mean ± SEM (n = 3–5).
Discussion
The primary finding from this study is that a decrease in atmospheric oxygen tension decreased the inflammatory response of human fetal membrane in vitro (Figures 1A and 2A). The effect on PGE2 seemed to last for 24 h, whereas IL-6 output was unaffected by this time point. It is, therefore, essential to consider whether 3% atmospheric oxygen represents normoxia or hypoxia for fetal membranes.
For the first 10 weeks of pregnancy, it is clear that the placenta, and presumably the whole conceptus including the fetal membranes, develops at a low oxygen tension (∼20 mmHg and ∼3% O2) (Jauniaux et al., 2000, 2001). By 12 weeks, the loss of cytotrophoblast plugs and perfusion of the placenta by maternal blood increase the local pO2 to ∼50 mmHg (8% O2), but the fetal membranes are not directly exposed to maternal blood, and hence may remain at a lower pO2. Direct support for this comes from measurements of oxygen in amniotic fluid; the pO2 remains between 10 and 30 mmHg throughout pregnancy (Quilligan, 1962; Vasicka and Hutchinson, 1964; Johnell and Nilsson, 1971). Blood flow in chorion is thought to be limited, so it seems likely that the amnion and chorion components of the fetal membranes normally will be exposed to low levels of oxygen. Decidua may have higher blood flow and hence oxygen, but the impact of this on the chorion and amnion is not clear. Indirect support for a low oxygen tension in fetal membranes comes from the presence of F. nucleatum (Cahill et al., 2005), which do not survive at O2 levels above 6% (Loesche, 1969). Overall, we suggest that 3% oxygen in vitro reflects in vivo conditions and, therefore, that consideration needs to be given to using a low oxygen tension for the culture of fetal membranes.
The second point from this study is that the process of inflammatory activation in fetal membranes, which leads to labour (Brown et al., 1998), leads to loss of tissue regulation by oxygen. In activated fetal membranes (Figures 1B and 2B), and in tissues obtained after labour (Figures 1C and 2C), oxygen was without effect on PGE2 or IL-6 output. We suggest that an oxygen-sensitive inhibitor is present in the non-activated tissues and that loss of this inhibitor during in vitro culture will lead to an increase in the response of tissue to IL-1β (Figures 1A and 2A). We would also suggest that this inhibitor is lost from activated and from post-labour fetal membranes, which gives rise to the high basal output of PGE2 from these tissues (Figure 1B and C); IL-1β had no effect, but the maximal ‘basal’ output would preclude in vitro stimulation by the interleukin. The data on IL-6 output from activated and post-labour tissues also do not fit the ‘loss of inhibitor’ hypothesis, as increased output of IL-6 in response to IL-1β was not found (Figure 2B and C), but there seems to be culture-independent change in IL-6 production so that IL-1β was completely without effect.
Oxygen-sensitive transcription factors have been investigated in recent years particularly in the context of hypoxic pathologies (Maxwell, 2005; Ruas and Poellinger, 2005; Pouyssegur et al., 2006) and to a lesser extent in inflammatory and other processes that involve reactive oxygen species (ROS) (Li and Nel, 2006). Most attention has been focused on the hypoxia-inducible factors (HIFs), a family of potent regulatory transcription factors (Maxwell, 2005; Ruas and Poellinger, 2005); although our data do not directly implicate HIFs in fetal membrane biology, many studies have shown that HIFs are important in placental development and function (Caniggia et al., 2000a, b; Rajakumar and Conrad, 2000). The chorionic component of human fetal membranes is contiguous with placental trophoblast, and we anticipate that chorion may express HIFs. Further studies are needed to confirm this hypothesis.
Our data suggest that a local increase in oxygen content in fetal membranes close to the time of labour could lead to a loss of inhibitor and the overall activation of inflammatory changes that have been reported (Kelly, 1996). There are no direct data on oxygen tension or blood flow in fetal membranes at such gestational ages, and data from amniotic fluid suggest that oxygen levels do not change close to labour (Quilligan, 1962; Johnell and Nilsson, 1971). It could be argued that increased production of ROS during the inflammatory process of labour (Kelly, 1996; Buhimschi et al., 2000) would regulate levels of inhibitor and hence increase the production of PGs and cytokines. Further work is needed to establish whether the inhibitory factor is lost before the inflammatory changes of labour are activated or afterwards. A precedent for such a generic change in biology has been shown in the case of type-2 cyclooxygenase expression and PG production (Brown et al., 1998), which are spontaneously and permanently activated in labour.
Acknowledgements
We thank Kidani Trust (ST) and the Institute of Obstetrics and Gynaecology Trust (MAA) for supporting this work.
References
Author notes
N.B. An error was made in the initial online pagination of Molecular Human Reproduction 13/3. The page span of this article was originally shown as 57–61. The publisher wishes to apologise for this error.